
Distek, Inc. Releases the sensIR 3200 Disintegrator
North Brunswick, NJ—December 2013—Distek, Inc., an industry leader in pharmaceutical laboratory testing instruments, accessories, and validation services, is pleased to announce the release of the sensIR 3200, their next generation “Bathless” Disintegrator.
The Distek sensIR 3200 uses the latest in bathless heating to offer a disintegrator without the mess and drawbacks associated with conventional water bath-based instruments. By eliminating the water bath and utilizing a sleek modular design, Distek’s sensIR 3200 offers two, four, or six disintegration test stations in the smallest footprint of any comparably equipped model.
The advanced features of the sensIR 3200 provide superior performance and functionality. With rapid media warmup, the sensIR 3200 heats media from ambient to 37 °C in less than eight minutes, significantly increasing throughput by offering rapid changeover between testing.
The sensIR 3200’s color touch-screen user interface was designed to provide the user with an intuitive and interactive tool to command the robust features. The icon-driven interface lowers overall cost by reducing training time and user errors.
The disintegration baskets can operate independently or in unison, thus increasing throughput. The sensIR 3200 meets or exceeds all harmonized compendial design requirements.
Available Q1 2014, the sensIR 3200 will offer Auto Endpoint Detection using “Patent Pending” Near Infrared. This state-of-the-art technology provides endpoint detection without the need of a modified fluted disk, therefore eliminating the accelerated disintegration and disparity the use of a fluted disk can cause.
Jeff Seely, Vice President of Sales & Business Development, says, “The release of the sensIR 3200 is another example of Distek’s innovation and leadership in the pharmaceutical testing market space. The use of IR to detect the presence of the tablet is a leap forward in disintegration testing providing reliable results and compliance with all monographs regardless if a fluted disk is required or not.”
For additional information on this or any of Distek’s industry leading products and services, contact Distek’s Corporate Office at +1 732 422 7585, by e-mail at info@distekinc.com, or visit www.distekinc.com
ABOUT DISTEK, INC.
Distek, Inc. (www.distekinc.com), headquartered in North Brunswick, NJ, is a leading manufacturer of pharmaceutical laboratory test equipment, specializing in dissolution products and services. Distek also provides solutions for UV fiber optics, media preparation, physical testing, disintegration, and validation services.
Distek, Inc.
Sean Gilmore
121 North Center Drive
North Brunswick, NJ 08902
T: 732 422 7585
F: 732 422 7310
E: info@distekinc.com
Distek, Inc. Releases the LabEye Digital Video System
North Brunswick, NJ—January 13, 2014—Distek, Inc., an industry leader in pharmaceutical laboratory testing instruments, accessories, and validation services, is excited to release the LabEye Digital Video System for Dissolution testing monitoring and recording.
The LabEye Digital Video System, engineered by Two- Square Science, streamlines pharmaceutical testing in both R&D and Quality environments. Designed specifically for laboratory use, the LabEye is a ready-to-implement multi-camera system. Both portable and scalable, the Lab- Eye performs visual process monitoring, chronicles your research in HD clarity, keeps your lab secure, and provides automation troubleshooting.
Jeff Seely, Vice President of Sales & Business Development, says, “The LabEye system is the result of a collaborative effort between Distek, TwoSquare Science, and industry leaders. The resulting system is a welcome and needed addition to any laboratory requiring process control and monitoring.”
Take the stress out of research and add productive time to your day. The LabEye provides scientists more free time while continuing to capture extremely important visual data. Optimize your time with remote viewing and continuous video recording capability.
For additional information on this or any of Distek’s industry-leading products and services, contact Distek’s Corporate Office at +1 732 422 7585, by email at info@distekinc.com, or visit www.distekinc.com.
ABOUT DISTEK, INC.
Distek, Inc. (www.distekinc.com), headquartered in North Brunswick, NJ, is a leading manufacturer of pharmaceutical laboratory test equipment, specializing in dissolution products and services. Distek also provides solutions for UV fiber optics, media preparation, physical testing, disintegration, and validation services.
Distek, Inc.
Sean Gilmore
121 North Center Drive
North Brunswick, NJ 08902
T: 732 422 7585
F: 732 422 7310
E: info@distekinc.com
Logan Instruments Corp. Launches Its Brand New 2-IN-1 DISSO III-7 Apparatus for both USP 3 AND USP 7
Somerset, NJ—Logan Instruments Corp., a leader in pharmaceutical testing instrumentation, released in December 2013 its latest version of USP Apparatus 3 and 7 combined in one single unit dubbed DISSO III-7.
Known for its premier instrumentation and advanced technological designs in transdermal testing, Logan Instruments has designed and marketed an individual Apparatus 3 (DISSO III Classic) and an Apparatus 7 (RRT- 7 Release Rate Tester) in the past. With limited choices, increasing demand, and economic practicality for USP Apparatus 3 and 7, Logan Instruments Corp. has created a new instrument that combines both USP methods with special features.
The DISSO III-7 offers the uniquely designed carried-over evaporation caps that prevent evaporation, a big concern in sustained-release dissolution using Apparatus 3, and help keep a uniform testing environment. Logan Instruments has improved its latest model by having a centered dipping arm to keep all sample shafts perpendicular to the outer tubes.
In order to run both USP testing methods, one only needs separate Dip Racks and Tube Carriers. Everything else remains the same with no additional interchangeable parts.
Finally, the DISSO III-7 can be automated with Logan Instruments’ new DSC-37 System Controller/SYR-8L-10ml Syringe Pump/SCT-240L Sample Collector, combined as the Automated System DISSO-III-7.

For more information, please contact us at info@loganinstruments.com
To learn more about Logan Instruments Corp.:
Logan Instruments Corp.Jensen Lee
19 C Schoolhouse Rd.
Somerset, NJ 08873
Office: 732-302-9888
Fax: 732-302-9898
www.loganinstruments.com
jensen@loganinstruments.com
Pion Launches the ΜFLUX™ - A Novel Dissolution-Permeability Apparatus
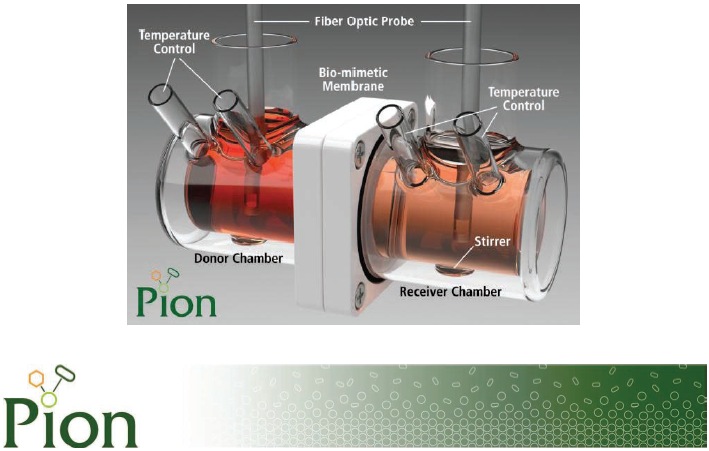
January 14, 2014—The ΜFLUX™ is Pion’s latest addition to the ΜDISS Profiler™, a fiber optic UV based in situ monitoring system. Its novel design allows users to conduct simultaneous dissolution-permeability assays. This type of experiment, previously very difficult and time consuming to perform, allows the user to immediately evaluate the absorption potential of an API or formulation by monitoring in situ concentration on both sides of a biomimetic membrane.
Pion’s Director of Science & Research, Dr Konstantin Tsinman, said, “ΜFLUX™ add-on to ΜDISS Profiler™ combines all benefits of in situ concentration monitoring with dissolution-permeability studies. Such combination provides a unique insight into dissolution/solubility and permeability interplay of complex formulations and allows predicting outcome of pharmacokinetic studies more accurately.”
About Pion Inc.
Pion Inc. develops and manufactures instrumentation for compound testing in pharmaceutical R&D. These include high-precision fiber optic-based analytical instruments for solubility and dissolution measurements, as well as complete systems for permeability (PAMPA), solubility, and ionization. Additionally, Pion provides CRO services for solubility, permeability, dissolution, pKa, lipophilicity testing, and excipient screening.
Contact Us
For more information, please visit www.pion-inc.com, call us at +1-978-528-2020 (x22), or send us an email at sales@pion-inc.com
Global Cooperation Between Takao Manufacturing Co. Ltd. and Dissolution Accessories
Takao Manufacturing Co. Ltd. (Japan) and Dissolution Accessories (The Netherlands) are proud to announce that both companies have signed an agreement making Dissolution Accessories the exclusive worldwide distributor of the Takao high precision dissolution vessel.
Takao provides dissolution vessels with the best precision, geometry, and repeatability one can find in a dissolution vessel.
Dissolution Accessories is a privately owned Dutch company who manufactures parts and consumables for a wide variety of dissolution instruments regardless of manufacturer. All Dissolution Accessories products are in compliance with USP, BP, EP, and JP guidelines.
Dissolution Accessories together with Takao Manufacturing Co. Ltd. has developed instrument-made vessels for a wide variety of brands, in standard and high precision. These vessels are available in most common dimensions in clear glass, amber glass, Peak, or Teflon coated. The vessels are adapted to every brand and work without the need of any separate adapter bracket; they fit directly in your dissolution instrument regardless of the manufacturer!
Pharmaceutical analysts worldwide may benefit from the Takao vessels performance, regardless of their vendor of choice.
The high precision vessels are customized to your request. We can produce these vessels at any time, changing their size, shape, or thickness to fully and immediately meet your testing requirements and specifications. Dissolution Accessories provides the vessels at an affordable price, despite its elaborate glass processing.
Dissolution Accessories offers the most extensive selection of glass vessels available from a single source. All vessels are now available!

For further reference, see:
Dissolution Technologies, 12(4) November 2005, pages 15-19.
Dissolution Technologies, 14(1) February 2007, pages 28-33.
For more information, please contact:
Mr. Adrie AldersPhone: +31-162-471485
adrie.alders@prosense.com
www.dissolutionaccessories.com
TWO SQUARE SCIENCE and VORTEX Announce Sales and Service Alliance
Fall River, MA, and Benson, NC—January 2, 2014—Two Square Science and VORTEX Sales Group, LLC, today jointly announced an agreement making VORTEX the exclusive sales, support, and service provider in the United States and Puerto Rico for Two Square’s CETUS Fully Automated Dissolution System for USP Apparatus 1 and 2.
The CETUS Dissolution System was developed through collaborations with leaders in the pharmaceutical AR&D and QC labs to streamline dissolution method development and formulation screening and improve efficiencies for final dosage form QC release testing. It is fast becoming the next generation in Automated Dissolution Systems for pharmaceutical companies in North America, Europe, and Asia.
The CETUS is a bench-top, traditional dissolution bath, designed to match more closely the standard dissolution testers on the market. It requires no robotic arms, no bulky basket stations, and no costly lab space modifications or special utility requirements.
“This is a major step forward in expanding the Cetus product line,” said Martin Schwalm, president of Two Square Science. “Our alliance with Vortex will enhance industry awareness and create a robust service backbone with new capabilities in application development.”
Stuart Bates, Director of Business Development at Vortex, noted that his company is uniquely qualified to fulfill this role: “The VORTEX team is a trusted partner in the dissolution lab, offering affordable and reliable service plans for maintenance, calibrations, and full service contracts for all brands of dissolution, GCs, HPLCs, and UVs. The CETUS offers unique advantages, and we bring to the table the ability to service and support these instruments as well as assist customers in method development, training classes, and SOPs for successful implementations.”

About Two Square:
Two Square Science designs and manufactures automated test and monitoring systems for the pharmaceutical, life science, and biotech industries. Our product line includes Fully Automated Dissolution Systems, Bench-top and Automated High-Speed Sample Preparation Systems, Automated NIR/Raman Systems, and Multi-Camera Lab-Compliant Video Monitoring Systems. Research and development is a continuing part of the Two Square Science story.

About VORTEX:
With facilities near the Research Triangle Park, NC, including a 4000 sq. ft. office, certification lab, warehouse, and support help-desk, our highly experienced and knowledgeable service and sales team comprise former dissolution and analytical vendors, dissolution chemists, and scientists from metrology departments of major pharmaceutical companies. We have staff and inventory in key parts of the country and can provide our customers guaranteed reaction time. Our goal is nothing less than exceptional service, support, and instrumentation. Learn more at www.vortexsg.com

Dissolution Testing of Capsules
March 24-25, 2014 • USP Headquarters, Meetings Center • Rockville, Maryland

Join USP for an interactive workshop on the challenges associated with developing dissolution procedures for capsules, specifically cross-linked gelatin capsules and capsules with lipophilic fillings. Discussion will focus on manufacturing, formulation, storage, and packaging conditions that could have an impact on the dissolution testing of any type of capsule (gelatin, starch derivatives, cellulose derivatives) containing any type of filling (solid, semisolid, liquid, dispersion, etc.).
The USP Dosage Forms Expert Committee has created Expert Panels to suggest improved compendial approaches to these challenges. These panels are also interested in concerns and quality attributes of capsules with shells made from other materials. USP activities regarding the revision of relevant USP-NF general chapters will also be discussed.
Topics Include
- Manufacture of hard and soft capsules, including nongelatin capsules and capsules containing lipophilic filling
- Causes of cross-linking in gelatin capsules (formulation, storage conditions, etc.)
- Modified-release capsules
- Use of enzymes in dissolution testing of cross-linked gelatin capsules
- Dissolution testing of dietary supplements
- Revisions to USP-NF General Chapters <711> Dissolution, <1094> Capsules— Dissolution Testing and Related Quality Attributes, and <2040> Disintegration and Dissolution of Dietary Supplements
- Validation and verification of dissolution procedures using enzymes
- Case studies
Who Should Participate
Scientists, managers, and other professionals who work in or with:
- Developing and validating dissolution tests
- Regulatory affairs
- Formulation
- Validation
- Pharmaceutical manufacturing
- Dietary supplements manufacturing
- Analytical chemistry
- Quality assurance/quality control
- Research and development
- Contract research organizations
- Contract analytical organizations
- Contract manufacturing organizations
REGISTRATION AND ADDITIONAL INFORMATION
Early registration and group discounts are available.
Website: uspgo.to/dissolution-capsules
Email: conferences@usp.org
Phone: +1-301-816-8136



